Why work with GD?
We don’t shy away from problems. Since 1973, our engineers have tackled the toughest challenges for the most rigorous environments, from the battlefield to the factory floor—and anywhere in between.
Industries
Over the decades, our team of hardware, software, and embedded systems engineers have solved thousands of problems in safety- and mission-critical industries:
Products
- Interactive Product Sampler
 PRODUCTS
PRODUCTSInteractive Product Sampler
ExploreBrowse our range of American-made workstations, displays systems, and other ruggedized electronics.
- Rugged Displays
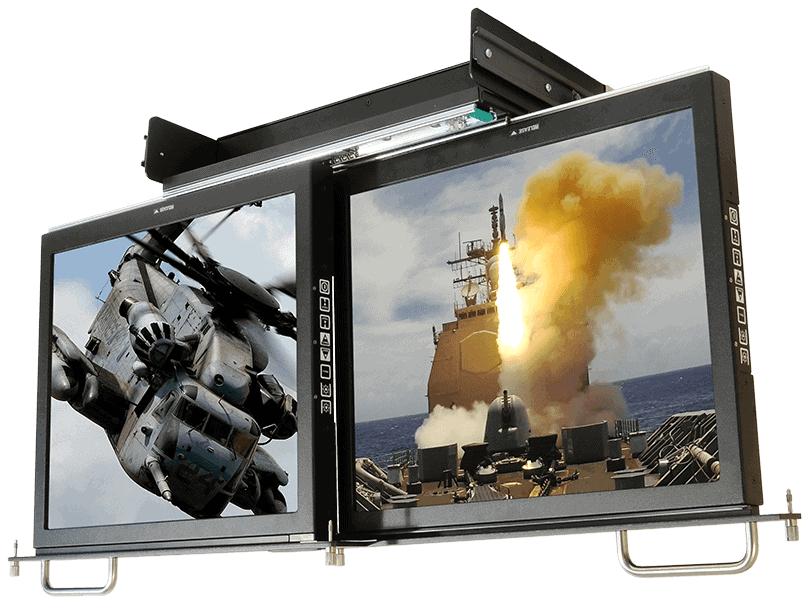 PRODUCTS
PRODUCTSRugged Displays
Learn MorePanel mount, rack mount, and standalone display solutions built to withstand the harshest environments from the factory floor to the battlefield.
- Keyboards
 PRODUCTS
PRODUCTSKeyboards
Learn MoreMilitary- and industrial-grade highly-customizable keyboards, including a variety of pointing devices, backlight colors, enclosures, interfaces, and more.
- Power Supplies
 PRODUCTS
PRODUCTSPower Supplies
Learn MoreRugged power supplies for extreme environments, including wide operating temperature ranges, shock and vibration protection, and EMI compliance to military standards
- Options & Accessories
 PRODUCTS
PRODUCTSOptions & Accessories
Learn MoreFrom video cables and touch screens to KVM switches and mounting arms, learn more about our complimentary options to ensure your display functions at peak performance.
- Display Enhancement Services
 PRODUCTS
PRODUCTSDisplay Enhancement Services
Learn MoreBrightness and NVIS enhancements, heaters, increased resistance to shock, vibration and moisture, and more to adapt your display to its environment.
- Commercial-grade Displays
 PRODUCTS
PRODUCTSCommercial-grade Displays
Learn MoreQuality, durable display systems for tough applications including outdoor signage and light-industrial environments
Services
- Independent Verification & Validation (IV&V)
 services
servicesIndependent Verification & Validation (IV&V)
Learn MoreUnit, Integration, and Systems testing for certified environments (DO-178, FDA, etc.) and other safety-critical applications
- Safety-Critical Software Development
 services
servicesSafety-Critical Software Development
Learn MoreFull Software Development Life Cycle solutions from planning to design to certification and post-launch.
- Application Development
 services
servicesApplication Development
Learn MoreCustomized application development for safety- and business-critical platforms, including Windows, Mac, Linux, iOS, Android, and RTOS.
- Agency Compliance & Certification
 services
servicesAgency Compliance & Certification
Learn MoreWork with industry experts on the planning and execution of your product’s regulatory submission.
- Product Evaluation & Modification
 services
servicesProduct Evaluation & Modification
Learn MoreRetrofit legacy equipment to be integrated with new or updated systems.